SaferLiver
With the SaferLiver solution package we provide services in the field of hepatoxocity including integrated in silico models and in vitro assays that are based on newest findings in hepatoxic molecular mechanisms (DILI) using newly developed cell and tissue models.
- About
- About
- Liver toxicity - an example
- Our solutions for you
- Our services
- Partner contribution
- 3D InSight™
- Transporter Modelling
- Silensomes™
- HepaRG™ cells
- Learn all about HepaRG™ cells
- What are HepaRG™ cells?
- Applications
- HepaRG profiling
- Products
- Tutorial and Support
- Regulatory framework
- Request further information
- Order links
About

Liver toxicity can be caused by chemicals through a wide range of mechanistic interactions. Mechanisms for liver toxicity include hepatocellular apoptosis, Kupffer cell activation (ROS, cytokine, and chemokine release), neutrophil infiltration, peroxy-nitrite production, cholestasis and mitochondrial dysfunction which constitute key events for liver toxicity adverse outcome pathways. Drugs and other chemical compounds are directly transported to and transformed in the liver after uptake where they are metabolized. It is an important goal to predict and avoid potential liver toxic effects during new product development. [1,2,3]
With the SaferLiver solution package we provide solutions to assess chemicals in concern of their hepatoxic potential including:
- Computational transporter models
- A kinetics data repository
- Next generation in vitro assays using the most innovative and hepatic cell line «HepaRG»
- 3D InSight Human Liver tissue models for Drug Induced Liver Injury (DILI) studies
- Silensomes™ which are human pooled liver microsomes (dP450(CYP))
- Multi organ on a chip technique to replace animal experiments
References
[1] Mechanisms of hepatotoxicity by Hartmut Jaeschke, Gregory J. Gors, Arthur I. Cederbaum, Jack A. Hinson, Dominique Pessayre and John J Lemasters 2002.
[2] From basic research to risk assessment by Shana J. Sturla, Alan R. Boobis, Rex E. FitzGerald, Julia Hoeng, Robert J. Kavlock, Kristin Schirmer, Maurice Whelan, Martin F. Wilks and Manuel C. Peitsch. 2014
[3] Current Concepts of Mechanisms in Drug Induced Hepatotoxicity by Stefan Russmann, Gerd A. Kullak Urblick and ignazio Grattagliano, 2009.
Liver toxicity - an example
Drug induced liver injury (DILI) is a major cause of liver dysfunction. It is divided into intrinsic and idiosyncratic hepatotoxicity. Intrinsic hepatotoxicity is predictable and is induced dependent on the dose of the compound exposed to the organism. Idiosyncratic hepatotoxicity on the other hand is not easy to predict. It is not dose dependent and leads to symptoms such as fever, skin reactions, eosinophilia, formation of autoantibodies and a short latency time particularly after re-exposure. DILI is underlied by complex molecular mechanism interactions which makes it challenging to describe it. To understand the induction of hepatotoxicity better, a three step model has been developed which visualizes the three main pillars of DILI and how it affects mitochondria function.
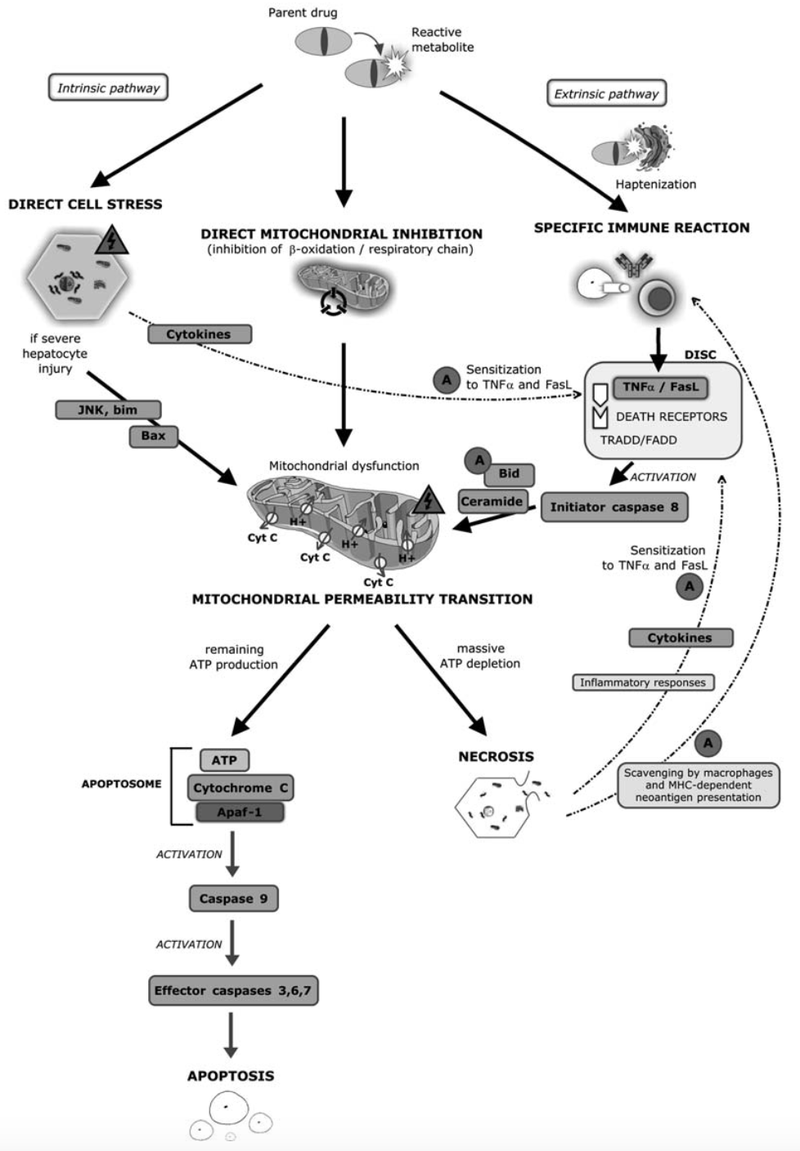
Figure1: Current Concepts of Mechanisms in Drug Induced Hepatoxicity. [1]
During risk assessment it is essential to identify specific key events that are based on the knowledge of underlying molecular mechanisms that lead to toxic effects. With our SaferLiver solution package we offer services which are specific for DILI and offer 3D InSight Human Liver tissue models which can be used for confident DILI studies. With this we predict possible side effects of compounds and suggest approaches on how you can design your products to be safe and sustainable.
Scientific related limitations
Although our services are based on the integration of current existing data in combination with assays for liver toxicity specific endpoints, we always keep up to date with newest findings in cutting edge science to identify new key events which we can implement into our services. Since new compounds and materials are being developed every day, such compounds might induce molecular mechanisms within the organism which scientists do not understand yet. The liver is the prioritized organ where potentially toxic compounds accumulate after entering the organism and where they might disrupt normal liver function. To ensure the safety of those compounds we have to better understand the underlying mechanisms to be able to always have the most innovative solutions ready with which we can support you in developing your safe and green product.
References
[1] Current Concepts of Mechanisms in Drug Induced Hepatotoxicity by Stefan Russmann, Gerd A. Kullak Urblick and ignazio Grattagliano, 2009.
Our services
Our services are based on an integrated and tiered strategy approach as it is shown in figure1. By accessing (omics) data and literature knowledge, we integrate them into our in silico modelling tools which are based on authority evaluation guidelines. With our tiered solutions we offer in silico characterisation and additionally in vitro and in vivo analysis if further data is needed. All results are implemented into our integrated data analysis where we generate results and offer suggestions which you can use to make your decision throughout your product design procedure. With this you can develop products with safer and greener ingredients.
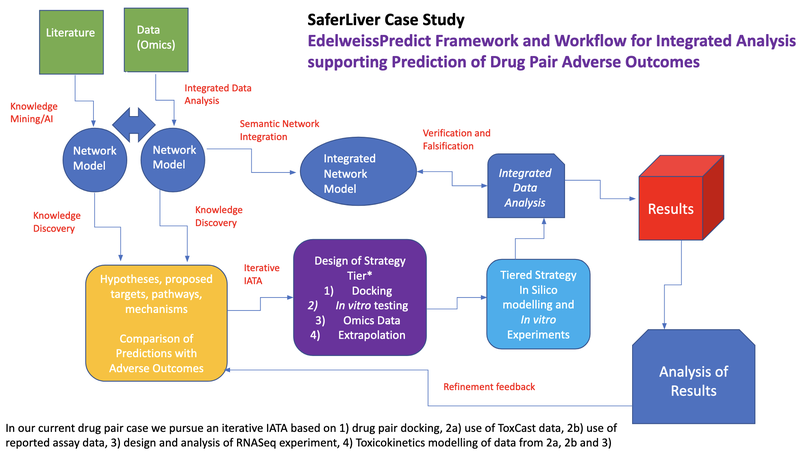
Figure1: EdelweissPredict Framework and Workflow for Integrated Analysis supporting Prediction of Drug Pair Adverse Outcomes.
Our approaches are based on the following methods:
In silico modelling, where we use existing data on known liver toxins that are evaluated by machine learning techniques by feature extraction and ontology characterization. The modles are impplemented in userfriendly and straightforward applications that allow effective characterization of compounds.
AI / Machine learning, that is based on QSAR computation. With our expertise we extract targeted knowledge from information collections (documents, abstracts etc.) and organize it as structured information e.g., available to applications from a customised EdelweissData™ instance.
Data Management, based on best data practises and satandards adhering to FAIR principles. Data and Metadata is harmonized, complete and safely managed in a cloud based system. Processing workflows are reproducible and easy accessible for future reference.
With this expertise we offer a strong and safe in silico solution for next generation risk assessment.
Partner Contribution
Together with our partners we offer solutions in next generation risk assessment that are based on newest technologies, including most advanced in silico machine learning modelling and data management and new developped cell and tissue models for advanced in vitro assays. With this we aim to help you to make your products safer and more sustainable. Our partner cotributions include:
Biopredic
Beside primary hepatocytes and diverse liver tissue samples, Biopredic provides their most innovative and useful hepatic cell line «HepaRG» with which it is possible to produce early hepatic progenitor cells as well as completely mature human hepatocytes.
> Read more
In Sphero
In Sphero has a profound liver toxicity expertise and offers customized solutions for your needs in liver toxicity characterization. This includes the offering of 3D InSight Human Liver tissue models which can be used for confident Drug Induced Liver Injury (DILI) studies to predict possible side effects of compounds. Furthermore, InSphero offers diverse assays including Omics profiling, inflammation mediated toxicity, CYP-mediated toxicity, Cholestasis, Drug-induced steatosis and apoptosis - assay to assist you in the development of your new safe and green product.
> Read more
Phenaris
Phenaris provides you with:
- Computational transporter models including data curation, model building and data analysis.
- ToxPHACTS including similarity search, data extraction and data analysis.
- A kinetics data repository including data collection, data integration and data analysis.
With this, you have an expertise which constantly challenges the status quo in computational drug design and in silico toxicology.
> Read more
TissUse
TissUse is a German based company which has developed a multi organ on a chip technique to replace animal experiments. Those chips can be used during drug development, in cosmetics, food, nutrition and consumer products to predict toxicity, ADME profiles and in vitro efficacy for example.
> Read more
Edelweiss Connect
Edelweiss Connect, as provider of the SaferWorldbyDesign platform, leverages its data expertise to assist in the modeling of liver toxicity. Using machine learning techniques Edelweiss Connect can help identify key markers and patterns that may show a substance’s potential to cause liver toxicity, allowing for better-informed decision-making in the drug development and risk assessment process.
> Read more
3D InSight™
3D InSight™ Human Liver Microtissues are prequalified, long-lived liver models designed for drug safety and efficacy testing, and the study of healthy and diseased liver function. Composed of the primary human liver cells necessary for core liver functions, these microtissues are delivered assay-ready, in a lab-automation-friendly plate format ideal for screening.
> Read more
Transporter Modelling
Transmembrane Transport Proteins (TMTs) control nutrient uptake, ion transport, and drug transport across biological membranes. Predicting substrate and inhibition profiles of small molecules towards these transporters helps medicinal chemists to prioritize compounds in an early phase of the drug development process and guide toxicologists in the safety assessment of candidate compounds.
> Read more
Silensomes™
Silensomes™ are human pooled liver microsomes in which cytochromes P450 (CYP) enzymes have been chemically knocked down, one per one. Silensomes™ is a unique solution for a direct quantitation of the fm (f for fraction, m for metabolized). Fm is a simple expression of the contribution of each CYP in drug metabolism of a test compound.
> Read more
HepaRG™ is a human bipotent progenitor cell line capable of differentiating into both biliary and hepatocyte lineages. It was originally derived from a female patient diagnosed with a cholangiocarcinoma and hepatitis C infection. See our «Learn all about HepaRG™ cells» section for further information.
About HepaRG™ cells
HepaRG™ is a human bipotent progenitor cell line capable of differentiating into both biliary and hepatocyte lineages. It was originally derived from a female patient diagnosed with a cholangiocarcinoma and hepatitis C infection. The HepaRG cell line, free of hepatitis B and hepatitis C viruses has the following main characteristics:
- HepaRG cells can undergo a complete program of hepatocyte differentiation until a fully adult hepatic phenotype
- Hepatocyte-like cells are polarized cells able to form bile canaliculi-like structures
- Hepatocyte-like cells breath aerobically, consume lactate and contain as many mitochondria as the human hepatocytes
- HepaRG cell line has the potential to express major properties of stem cells: high plasticity & transdifferentiation capacity
The cells express the major nuclear receptors referred also as xeno-sensing receptors (Pregnane X receptor (PXR), constitutive androstane receptor (CAR), aryl hydrocarbon receptor (AHR)) (Figure 1, Antherieu et al., 2010 Aninat et al., 2005), drug and bile acids transporters (NTCP, BSEP, MRP2, MRP3, MRP4, MRP5, OSTa/b, MDR1, MDR3 etc.) (Figure 1 and 2, Bachour-El Azzi et al., 2015 and Anthérieuet al., 2010) and functional levels of phase I (CYP (CYP1A1/2, CYP2B6, CYP2Cs, CYP3A4 etc.) (Jinpeng Li et al. 2019) and II (UGT1A1, GSTA1, GSTA4, GSTM1) drug metabolizing enzymes (Figure 1, Anthérieu et al. 2010) as well as key hepatic nuclear factors such as Hepatocyte Nuclear Factor 4 (HNF4).
Furthermore, HepaRG™ cells show functional mitochondria, hepatokine secretion abilities and an adequate response to insulin (Figure 3). The HepaRG™ cell system appears as a robust surrogate for primary hepatocytes, which is versatile enough to study not only xenobiotic detoxification, but also the control of hepatic energy metabolism, secretory function and disease-related mechanisms (Tascher et al. 2019).
Unlike primary human hepatocytes, fully differentiated HepaRG cells can survive up to 4 weeks in culture allowing long-term studies and repeated exposures to drugs and chemicals (Figure 4, Jolly et al., 2020).
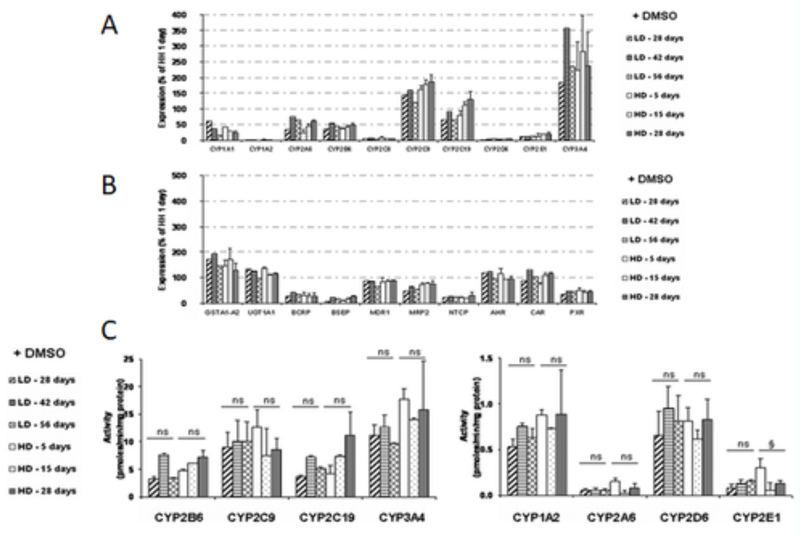
Figure 1: Basal mRNA levels of phase I and II metabolizing enzymes, membrane transporters, and nuclear receptors (A and B) and comparative basal activity levels of P450s (C) in HepaRG cells seeded at low density (LD) and cultured for 5 to 56 days or at high density (HD) and cultured for 5 to 28 days in presence of DMSO. Results are expressed as percentages compared with 1-day cultured human hepatocytes, arbitrarily set at 100% (Anthérieu et al., 2010).
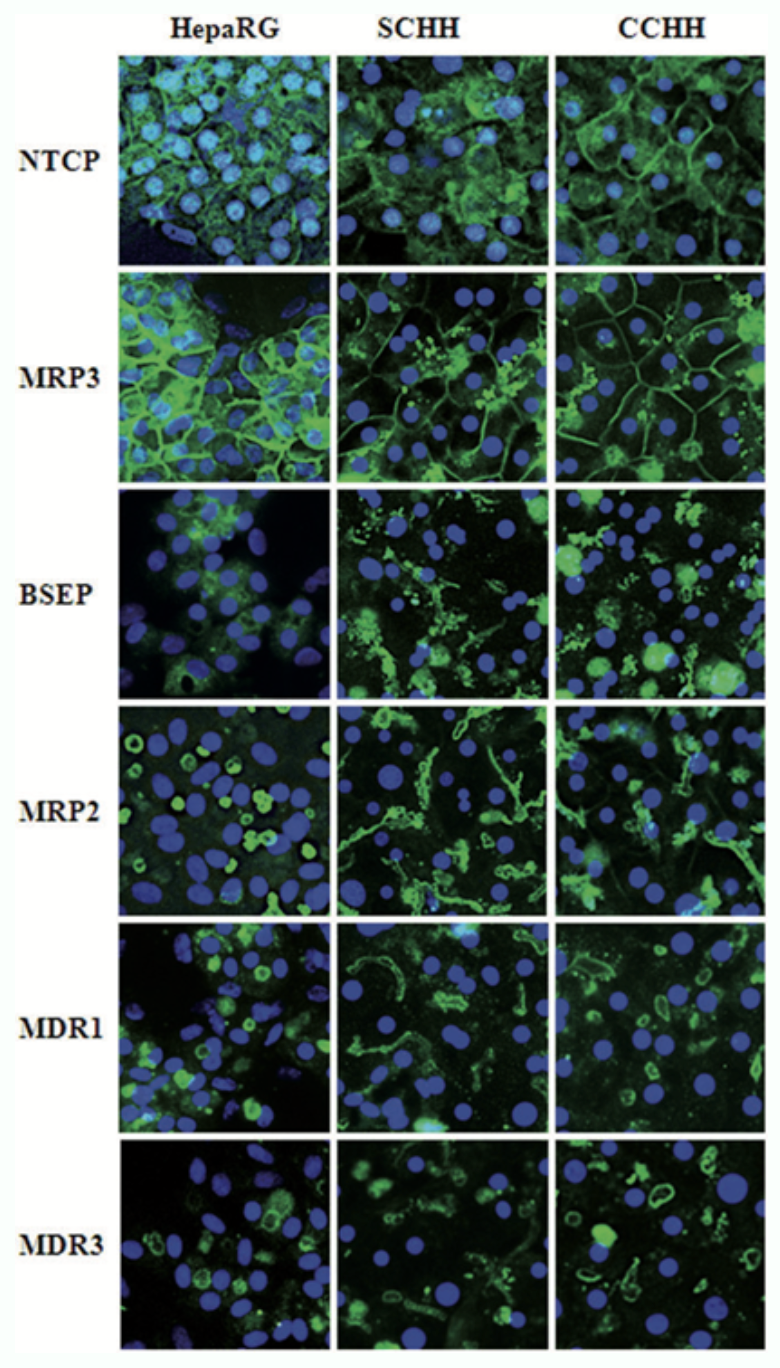
Figure 2: Distribution of main bile acid transporters in HepaRG cells and HHs.
Localization of several main hepatobiliary transporters was assessed by immunofluorescence staining in HepaRG cells as well as in 4–5 days HHs in sandwich (SCHH) and conventional (CCHH) cultures. Nuclei were labeled using Hoechst. Transporter immunolabeling in HepaRG cell cultures was restricted to hepatocyte-like cells; no transporter was visualized in primitive biliary cells (Bachour-El Azzi et al., 2015).
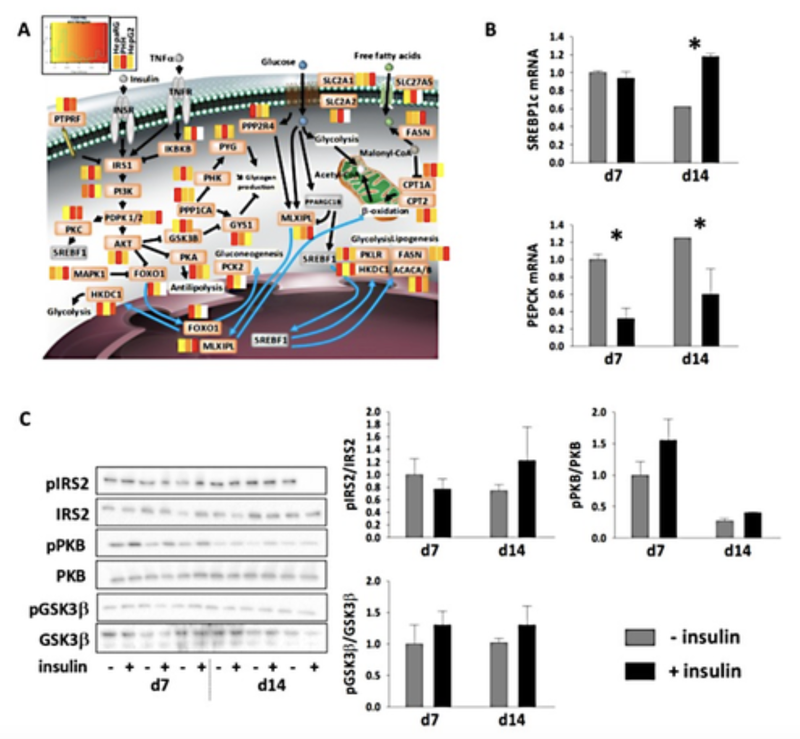
Figure 3: Insulin metabolism in highly differentiated HepaRG™ cells.
(A) The relative difference of protein abundances in HepaRG™ (defined serum-free medium: 0.5% DMSO, growth factor supplementation) and in HepG2 cells versus PHH, as shown as heatmaps for factors of insulin signaling/resistance pathways (white boxes: proteins not seen). Non-detected proteins are colored in grey. (B) mRNA levels of SREBP1c and PEPCK in differentiated HepaRG™ cells (differentiation medium: 1.7% DMSO, 10% FCS) in response to insulin stimulation (means ± SEM of three determinations). * indicates significant differences due to insulin stimulation for a given time point (ANOVA and post hoc Tukey tests; p < 0.05). (C) Phosphorylation status of IRS2, PKB, and GSK3β in differentiated HepaRG™ cells in response to insulin stimulation (means ± SEM of three determinations). (Tascher et al. 2019)
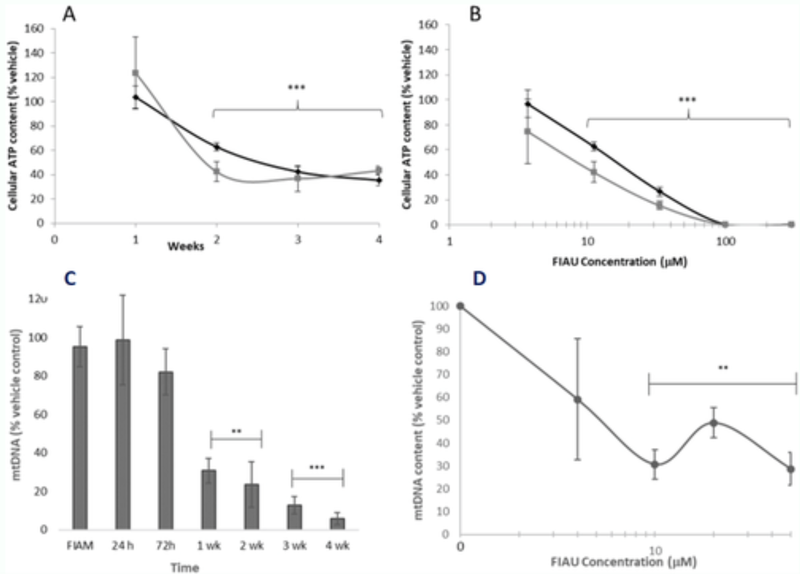
Figure 4: The utility of HepaRG cells in investigating the delayed toxicity.
Cellular ATP content after metabolic modification assay performed in HepaRG cells exposed to FIAU (0–300 μM). A: Time-response curve following exposure of HepaRG cells to 11 μM FIAU. B: Concentration-response curve following 2 weeks exposure of HepaRG cells. Round black markers represent data collected in glucose media, square black markers represent data collected in galactose media
Mitochondrial DNA content in HepaRG exposed to 10 μM FIAU (0–300 μM, 1–4 weeks). C: Time-response curve of FIAU following exposure to 10 μM FIAU or FIAM (4 weeks). B: Concentration-response curve following 2 weeks exposure to FIAU. Significance of each data point compared to vehicle control; ** p < .01, *** p < .001 (Jolly et al., 2020).
The process of HepaRG differentiation
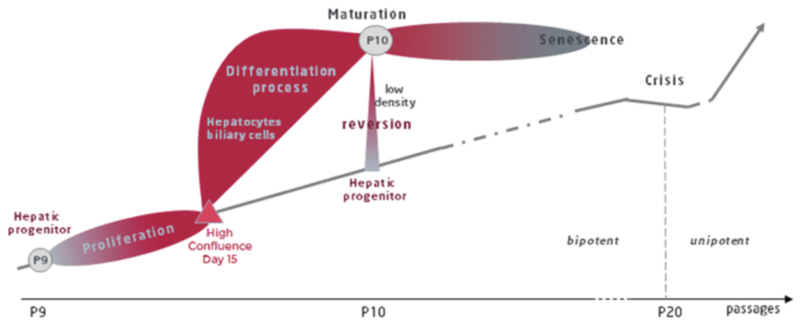
Two main stages are being identified, namely:
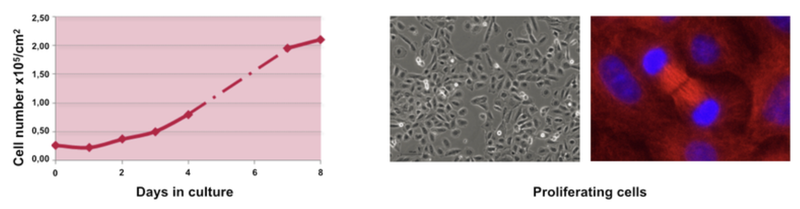
The proliferation stage
During the proliferation stage, the cell doubling time is 24h. At day 14, cells reach high confluence. At this moment the cells can be either split to re-enter proliferation stage or start the next differentiation stage.
The differentiation stage
To undergo hepatic differentiation, the cells are shifted to a medium enriched supplemented with 1.7% DMSO for a 14-day period. Within 3-4 days, two distinctive cell populations are recognized: one corresponding to hepatocyte colonies and the other one corresponding to primitive biliary cells. Once fully differentiated, the hepatocyte-like cells express a full array of functions, responses, and regulatory pathways of primary human hepatocytes including: Phase I and II metabolizing-enzymes and transporter activities consistent with those found within a population of primary human hepatocytes including intact response elements, PXR, CAR and PPARa.
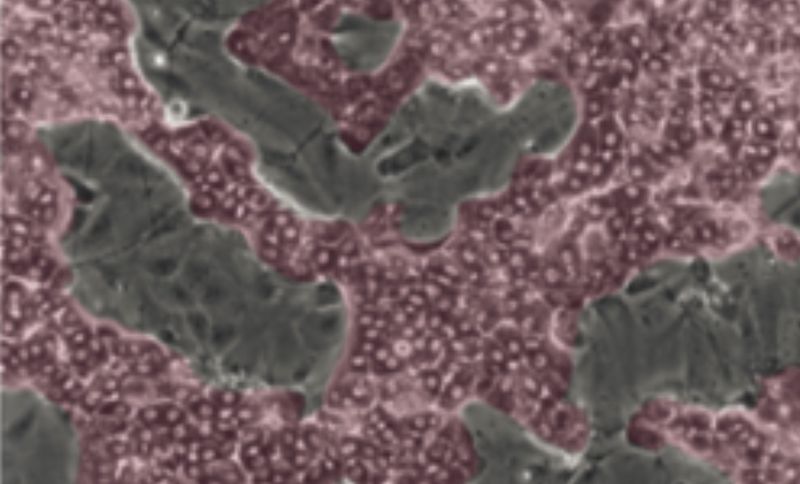
Confluent HepaRG™ cells start to commit into either hepatocyte (coloured in red) or biliary cells (coloured in grey).
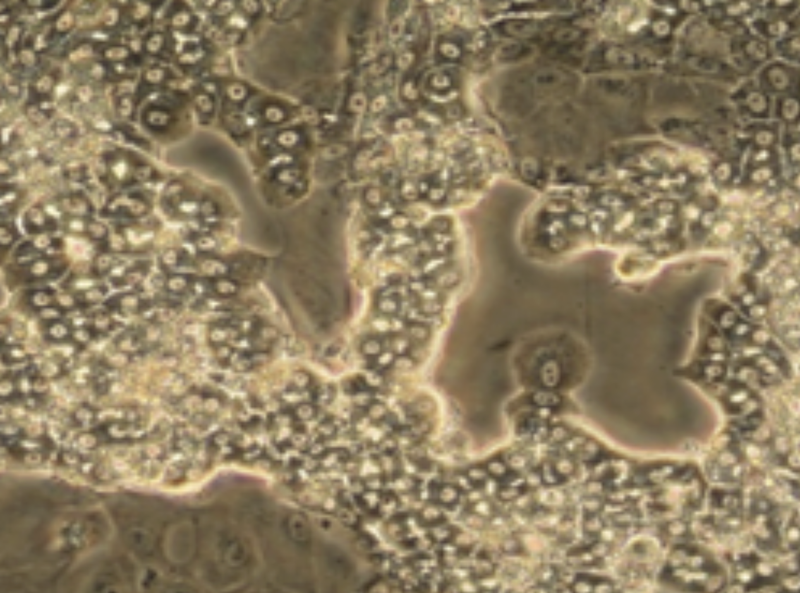
After 2 weeks, a mixed population of 2 types of cells, namely hepatocyte-like colonies surrounded by clear epithelial cells corresponding to primitive biliary cells, form a confluent monolayer which can be maintained in this stable form for several weeks in the presence of 1.7% DMSO.
Applications
HepaRG™ can be used in various applications including in vitro ADME testing, inhibition assays, drug metabolism and clearance, cholestasis, virology (HBV, HCV) and viral infection. Below, a few examples of applications with references are listed.
> The HepaRG utility in a NAFLD investigation
> CYP induction In-vitro ADME
Cytochrome P450 induction assay on HepaRG cell model
Drug-induced liver injury (DILI) and drug-drug interactions (DDIs) are concerns when developing safe and efficacious compounds.
HepaRG cells have a full complement of drug metabolising enzymes (DME, Phase I and II), drug transporters and nuclear receptors (CAR, PXR, and AhR). So that, HepaRG cell model, recognized by the FDA (Guidance for inductry 2006), can be used as a single batch or donor of human hepatocytes for determining if the Investigational drug is an Inducer of metabolizing enzymes in vitro. In addition, the variation in induction responses was very high in primary human hepatocytes; whereas, values for HepaRG cells were reproducible. The CYP induction responses in HepaRG cells are well within the range exhibited by primary human hepatocytes and can therefore be considered to be representative of a typical primary human hepatocyte (PHH) donor that can be used in multiple induction experiments conducted over years to generate reproducible control data.
Area:In-vitro ADME Cell culture type:2D Publication:
- In Vitro Drug Interaction Studies-Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions. FDA Guidance for industry https://www.fda.gov/regulatory-information/search…
> Cholestatic Hepatotoxicity/DILI Hepatotoxicity screening and mechanistic testing
Prediction of drug-Induced Intrahepatic cholestasis by measuring early alterations of bile canaliculi dynamics in HepaRG cells
Cholestasis is defined as the decrease or suppression of bile flow due to impaired secretion by hepatocytes or to obstruction of bile at any level of the excretory pathway. A key functional parameter for good bile flow from hepatocytes is contraction of the bile canaliculi. Evidence shows that cholestatic drugs consistently cause an early alteration of bile canaliculi dynamics associated with modulation of ROCK/MLCK and these changes are more specific than efflux inhibition measurements alone as predictive nonclinical markers of drug-induced cholestasis. Models and methods for detecting and characterizing drug-associated cholestatic disorders have been attempted. One problem relates to finding suitable hepatic models for in vitro studies. Hepatocyte polarization with bile canalicular formation is a complex mechanism which includes cytoskeletal, tight junctional and intracellular trafficking components. HepaRG cells which express phases 1 and 2 drug metabolizing enzymes and transporters, and form bile canaliculi structures, can be used to mimic features of intrahepatic cholestasis induced by cholestatic drug treatment and to characterize the mechanisms involved in the initiation of the lesions. Since it has been demonstrated that 12 tested cholestatic drugs could cause bile canaliculi constriction and dilatation in HepaRG cell cultures associated with different disturbances of the ROCK/MLCK. All fluorescent marker compounds driven to the biliary pole by transporters can be used as well for High Content Screening with imaging analysis. Exemplary assay is performed using CDFDA as marker compound for detecting the bile canaliculi deformation resulting in constriction or dilatation of the canalicular lumen. All those data suggest that HepaRG cells represent a suitable, easy-to-use liver model for identification of drug-induced cholestasis. In addition, the early alterations of bile canaliculi dynamics associated with Rho kinase/myosin light chain kinase pathway are the new potential predictive biomarkers of drug-induced cholestasis using HepaRG.
Area:Hepatotoxicity screening and mechanistic testing Cell culture type:2D Publication:
- Sharanek A, Burban A, Burbank M, Le Guevel R, Li R, Guillouzo A, Guguen-Guillouzo C.: Rho-kinase/myosin light chain kinase pathway plays a key role in the impairment of bile canaliculi dynamics induced by cholestatic drugs.. Sci Rep. 2016 May 12;6:24709. https://doi.org/10.1038/srep24709
> Mitochondrial toxicity detection Hepatotoxicity screening and mechanistic testing
Functional Mitochondrial Toxicity Assay on HepaRG cells using Seahorse XFe 96 flux analyser
Drug-induced liver injury (DILI) and drug-drug interactions (DDIs) are concerns when developing safe and efficacious compounds. HepaRG cells have a full complement of drug metabolising enzymes (DME, Phase I and II), drug transporters and nuclear receptors (CAR, PXR, and AhR). So that, HepaRG cell model, recognized by the FDA (Guidance for inductry 2006), can be used as a single batch or donor of human hepatocytes for determining if the Investigational drug is an Inducer of metabolizing enzymes in vitro. In addition, the variation in induction responses was very high in primary human hepatocytes; whereas, values for HepaRG cells were reproducible. The CYP induction responses in HepaRG cells are well within the range exhibited by primary human hepatocytes and can therefore be considered to be representative of a typical primary human hepatocyte (PHH) donor that can be used in multiple induction experiments conducted over years to generate reproducible control data.
Area:Hepatotoxicity screening and mechanistic testing Cell culture type:2D Publication:
- Mathieu Porceddu, Nelly Buron, Pierre Rustin, Bernard Fromenty, Annie Borgne-Sanchez: In vitro assessment of mitochondrial toxicity to predict drug-induced liver injury. Drug-Induced Liver Toxicity pp 283-300 https://doi.org/10.1007/978-1-4939-7677-5_14
> Repeated exposure Hepatotoxicity screening and mechanistic testing
HepaRG cells for in vitro chronic toxicity studies
Drug-induced liver injury (DILI) is a major cause of attrition during both early and late stages of drug development and a leading reason for drug withdrawal in postmarketing. Hepatocytes constitute approximately 80% of total liver mass making drug-induced hepatotoxicity a serious concern for the pharmaceutical industry.
Current models to predict hepatotoxicity in preclinical stages have shown many limitations. Interspecies differences in expression and activities of enzymes and transporters involved in drug metabolism and pharmacokinetics are a major limitation of animal models. The use of primary human hepatocytes, the gold standard for studying hepatotoxicity, is limited by the lack of availability, the interdonor variability and the rapid loss of hepatic functions that makes them unsuitable for long term hepatotoxicity prediction. Although 3D cultures retain their molecular phenotypes and hepatic functions for multiple weeks in culture, their predictive capacity of hepatotoxicants has not been fully assessed and their use for high throughput screening is not yet optimized.
Differentiated HepaRG cells express the main hepatic functions, including phase I and II enzymes, transporters, and nuclear receptors at levels comparable to those found in primary human hepatocytes and maintain this expression almost stable over 4 weeks. The ability of HepaRG cells to remain differentiated in culture over extended period give this model a definite advantage of studying the delayed onset of hepatotoxicity. HepaRG cells represent a unique metabolically competent cell model for in vitro chronic toxicity studies. This model has proven high sensitivity, specificity, and accuracy of flagging human hepatotoxic compounds. The utility and performance of HepaRG are particularly highlighted in the investigation of delayed hepatotoxicity.
Area:Hepatotoxicity screening and mechanistic testing Cell culture type:2D Publication:
- Jolly CE, Douglas O, Kamalian L, Jenkins RE, Beckett AJ, Penman SL, Williams DP, Monshouwer M, Simic D, Snoeys J, Park BK, Chadwick AE: The utility of a differentiated preclinical liver model, HepaRG cells, in investigating delayed toxicity via inhibition of mitochondrial-replication induced by fialuridine. Toxicol Appl Pharmacol. 2020 Sep 15, 403:115163 https://doi.org/10.1016/j.taap.2020.115163
> BSEP inhibition Hepatotoxicity screening and mechanistic testing
BSEP inhibition for in vitro screening cholestatic potential of drugs
BSEP (bile salt export pump), located on the canalicular membrane of hepatocytes, is the major efflux transporter for the secretion of bile acids (BA) from hepatocytes into bile in humans. Because that bile secretory failure via BSEP inhibition can lead to cholestatic DILI in humans, the European Medicines Agency Guideline on the Investigation of Drug Interactions (2012) recommends in vitro screening of BSEP inhibition!. Differentiated HepaRG hepatocytes are highly polarized cells exhibiting specialized apical (canalicular) and sinusoidal (basolateral) domains. As well HepaRG cells express canalicular transporters, including BSEP, MRP2, MDR1, and MDR3. Therefore, HepaRG cells could be useful to mechanistically better understand drug-induced alterations of BA influx and efflux. In addition, two novel bile acid fluorescent probed are synthesized by Starlight which are potential specific for BESP transporter. The inhibition of BSEP in HepaRG cells by incubation of candidate drugs and BA fluorescent probes result in a decreased accumulation of BA fluorescent probes inside the bile canaliculi networks in HepaRG cells. Therefore, measurement of BSEP inhibition on the efflux of fluoresence probes by candidate drugs allow to predict BSEP-drug candidate interactions. HepaRG cells represent a suitable model for studying hepatobiliary transporters and drug-induced cholestasis.
Area:Hepatotoxicity screening and mechanistic testing Cell culture type:2D Publication:
- Pamela Bachour-El Azzi, Ahmad Sharanek, Audrey Burban, Ruoya Li, Rémy Le Guével, Ziad Abdel-Razzak, Bruno Stieger, Christiane Guguen-Guillouzo: Comparative Localization and Functional Activity of the Main Hepatobiliary Transporters in HepaRG Cells and Primary Human Hepatocytes. Toxicol Sci. 2015, 145(1): 157–168. https://doi.org/10.1093/toxsci/kfv041
> 3D HEP/HSC systems for toxicity assessment and liver fibrosis modeling In-vitro ADME
Generation of 3D liver system with HepaRG/stellats co-culture for toxicity assessment and liver fibrosis modeling in vitro
A new proof of principle for the use of HeapRG-HSCs to generate complex in vitro spheroid cultures that better mimic the complexity of the liver as well as liver function. In this 3D co-culture, HepaRG-HSCs promote maintenance of hepatocyte metabolic functionality while being able to respond to hepatocyte-mediated toxicity, activating and promoting intra-spheroid fibrogenesis, one of the main drug-associated adverse liver outcomes. Since the potential use of HepaRG/HSC spheroids for the assessment of fibrogenesis is evaluated by exposure of the spheroids to TGF-b and thioacetamide (TAA) and measure of the expression of fibrogenic markers and secretion of pro-collagen type I and increased phalloidin and collagen staining. This relevant human hepatic organoid model is suitable for testing drug-induced liver fibrosis and liver fibrosis modeling in vitro.
Area:In-vitro ADME Cell culture type:3D Publication:
- Sofia B Leite, Tiffany Roosens, Adil El Taghdouini, Inge Mannaerts, Ayla J Smout, Mustapha Najimi, Etienne Sokal, Fozia Noor, Christophe Chesne, Leo A van Grunsven: Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials, 2016, 78, 1-10 https://doi.org/10.1016/j.biomaterials.2015.11.026
HBV/HDV infection Infectious diseases Use of HepaRG cell line for studies of HBV/HDV infection
The human liver cell line HepaRG, after differentiation, resembles PHHs with respect to many hepatocyte-specific markers including the expression of cytochrome P450 enzymes, liver-specific transcription factors, and transporter proteins like the HBV-specific receptor, sodium taurocholate co-transporting polypeptide (NTCP). So far, the HepaRG cell line is the only one allowing both studies on HBV/HDV infection and liver-specific drug toxicity and metabolism. So that HepaRG cell line is used as a suitable in vitro cell culture systems for investigations of virus-host interactions becouse of its efficiently support virus infection.
Area:Infectious diseases Cell culture type:2D Publication:
- Yi Ni, Stephan Urban: Hepatitis B Virus Infection of HepaRG Cells, HepaRG-hNTCP Cells, and Primary Human Hepatocytes. Hepatitis B Virus pp 15-25 https://doi.org/10.1007/978-1-4939-6700-1_2
> FADU assay for genotox risk assesment in human HepaRG cells In-vitro ADME
Automated Flurorimetric Detection of Alkaline DNA Unwinding on the metabolically competent HepaRG 2D culture
FADU assay is designed for automated genotox risk assessment via DNA strand break analysis in one 96-well plate of adherent HepaRG cell culture. The DNA damage detection id based on progressive DNA unwinding under highly controlled conditions of alkaline pH, time and temperature. A fluorescent dye id used as marker for double stranded DNA and decrease in the flurescence intensity indicates an increase in DNA unwinding and consequently a greater number of DNA straind breaks. Controls are performed in parallel with the experimentally treated cells.
Area:In-vitro ADME Cell culture type:2D Publication:
- María Moreno-Villanueva , Tobias Eltze, Dirk Dressler, Jürgen Bernhardt, Cordula Hirsch, Peter Wick, Gudrun von Scheven, Kirsten Lex, Alexander Bürkle: The automated FADU-assay, a potential high-throughput in vitro method for early screening of DNA breakage.. ALTEX. 2011;28(4):295-303 https://doi.org/10.14573/altex.2011.4.295
HepRG profiling
Gene expression profiling of the HepaRG cells (8 Days after seeding and respectively 24h in induction media)
Available products
HepaRG™ cells
The cells can be purchased as fully differentiated cells or undifferentiated growth-stage cells.
Fully differentiated cells: HPR116
Fully differentiated cells have characteristics closely related to those of primary hepatocytes. Advantages:
- Ready and easy-to-use model;
- High interassay reproducibility;
- Time-saving and efficiency;
- Suitable for high-throughput applications;
- Functionality across multiple applications;
- Cost-effective compared to primary human hepatocytes;
- No need of particular equipment;
HepaRG™ cells, catalog number HPR116 are cryopreserved after differentiation using a proprietary process developed by Biopredic International. A few days after cell thawing and culturing, a coculture of hepatocytes and of biliary epithelial-like cells is obtained.
> Visit shop to order HPR116 cells
Undifferentiated growth-stage cells: HPR101
Undifferentiated growth-stage cells can be grown in-house to set-up a master and/or working bank or further differentiated following the recommended procedures. Advantages:
- Possibility of cell line manipulation such as transfections, gene editing, etc;
- A proliferating cell population, adequate for some particular applications (e.g., Micronucleus test);
- Karyotypic stability: can be grown and split up till eight times when done according to the recommended protocols and using appropriate medium supplements;
- Possibility of cell line amplification
- Cells in proliferation stage tolerate some viral infections more easily than differentiated, adult hepatocytes.
HPR101 are cryopreserved undifferentiated HepaRG cells at passage 12. These cells are intended to be expanded and differentiated in-house. The undifferentiated HepaRG cell line is available only after a license agreement made with Biopredic International (for profit customers) or under a MTA made with Biopredic International (for non-profit customers that are not funded neither in full nor in part by a profit organization).
> Visit shop to order HPR101 cells
Media, supplements and plates
The plasticity of HepaRG cells is a property which can easily lead to cells phenotypically different from those described in the literature when HepaRG cells are not cultured and maintained according to the recommendations and under the appropriate conditions.
We recommend using our cell culture media and supplements with containing very carefully selected components. The formulation of our cell culture media and supplements ensures a correct culture of HepaRG cells leading to fully differentiated hepatocyte-like cells.
Our media rely on supplements (called ADDs) adapted to the phase stage of the HepaRG™ cell culture and to the usage intended use of the cells. Those ADDs are stored frozen and added to a basal hepatic cell culture medium.
Tutorials and support
Protocols
Cell thawing, viability measurement and counting, seeding and maintenance protocols for HPR116 - Differentiated HepaRG™ cells.
> Learn more
Training
We offer customized training on in vitro cell systems or in silico services, data analysis and management and tutorials.
> Learn more
Contact us
For more support on the HepaRG™ cell line please write to us.
> Contact us
Regulatory Framework
To guarantee the reliability of our characterization and assessment models, scientific reproducibility and data integrity have highest priority in our solution implementation. This approach not only underlies our methodology aligned with the current guidelines on liver safety assessment, but also takes into account what emerging regulatory guidelines will include based on new scientific progress.
- USA, Environmental Protection Agency, Framework for Cumulative Risk Assessment, EPA/630/P-02/001F, May 2003.
Reference:ADD610C
Vendor:Biopredic International
Product: HepaRG® Pre-induction Medium Supplement with antibiotics
Reference:ADD620C
Vendor:Biopredic International
Product: HepaRG® Maintenance/Metabolism Medium Supplement with antibiotics
Reference:ADD621C
Vendor:Biopredic International
Reference:ADD640C
Vendor:Biopredic International
Product: HepaRG® Induction Medium Supplement with antibiotics
Reference:ADD650C
Vendor:Biopredic International
Product: HepaRG® Serum-free Induction Medium Supplement with antibiotics
Reference:ADD651C
Vendor:Biopredic International
Reference:ADD670C
Vendor:Biopredic International
Product: HepaRG® Thawing/Plating/General Purpose Medium Supplement with antibiotics
Reference:ADD710C
Vendor:Biopredic International
Reference:ADD720C
Vendor:Biopredic International
Product: HepaRG® Differentiation Medium Supplement with antibiotics
Reference:HPR101
Vendor:Biopredic International
Reference:HPR109
Vendor:Biopredic International
Reference:HPR116080
Vendor:Biopredic International
Reference:HPR116100
Vendor:Biopredic International
Reference:HPR116120
Vendor:Biopredic International