SaferSkin
SaferSkin™ is an integrating application which supports the determination of key safety properties of skin, including irritation, tolerance, penetration, metabolism and sensitization. SaferSkin™ integrates in silico and in vitro methods and services in one application, supported by a harmonised data infrastructure and an expert consulting team.
- About
- Our solutions for you
- Our services
- Partner contribution
- In vitro testing
- SaferSkin Formulation testing
- SaferSkin case studies
- Prediskin
- SaferSkin Application
- SaferSkin application
- Regulatory framework
- Request further information
- Order links
About
SaferSkin™ is an integrating application that supports the determination of key safety properties of skincare products, including irritation, tolerance, penetration, metabolism, and sensitization.
Our mission is to ensure consumer safety by identifying potential dangers in your chemical formulations by providing comprehensive information about your finished products.
We provide step-by-step assistance for your projects in cosmetic and pharmaceutical development – while maintaining full compliance with all regulatory requirements.

SaferSkin Workflow - An example
Our solutions are based on authority guidelines which define the workflow during assessment to guarantee data integrity. The SaferSkin (OECD TG 497) decision tree serves as an example of such a workflow for skin safety risk assessment. It is well described in the guideline and includes the results of three standardized in vitro methods «DPRA», «KeratinoSensTM» and «h-CLAT». Each assay is specific for a molecular key event leading to skin sensitization. Two out of the three assays have to give a clear result, otherwise the results are considered inconclusive and further data has to be included for evaluation.
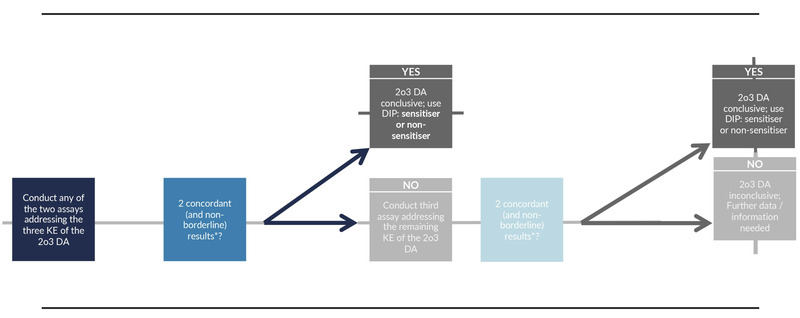
Note: Borderline results are determined based on workflows given in Annex 1 of the OECD TG 497 guideline:
«The use of information elements is dictated by the limitations as found in the respective test guidelines (TG 442C, Appendix 1; TG 442D, Appendix 1A; TG 442E, Annex 1). For example, in case a negative h-CLAT result is obtained for a chemical with Log P > 3.5 (according to the limitation described in TG 442E (4)), a 2o3 DA prediction can only be made if the outcomes of the other two test methods composing the 2o3 DA are concordant and are non-borderline.»
SaferSkin Case Studies
Included in our offers are case studies where we evaluate compounds based on an internal procedure that includes in silico and in vitro approaches based on the authority guidelines. With the following link we provide several examples of case studies including Defined Approaches (DA) from the OECD 497 guideline.
> Here are several case studies where we compared the results of different approaches including DAs from OECD TG 497.
Our services
Through our integrated approach, we collect and curate background data and apply algorithms including all main industry-led and OECD Defined Approaches (DA) supporting model predictions and comparisons – a process that leads to safer, greener, and more sustainable products.
What we provide
- Formulation and ingredient modelling, testing and assessment
- Expert study design, consulting and regulatory reporting
- Tiered strategy of in silico and in vitro based modelling and assessment for skin irritation, penetration and sensitization
- In vitro skin sensitization assay testing according to OECD guidelines and GLP standards (DPRA, KeratinoSensTM, h-CLAT)
- In silico based modelling using defined approaches according to industry best practices
- Advanced omics-based assay testing of ingredients and formulations
- Evaluation of advanced materials including nanoforms
- High quality harmonized data integrity and provenance supported by EdelweissData™
Those services are based on the following methods:
In silico modelling
Our in silico modelling and in vitro testing workflows are anchored to key events of Adverse Outcome Pathways (AOPs).
We can apply a variety of algorithms and machine learning techniques to SaferSkin™ datasets supporting the building of reference and customized models.
For skin sensitization we have reproduced the leading defined approaches developed and used at industry leaders such as P&G, BASF, Givaudan and Shiseido and including a well-documented and easy-to-use comparison of model results.
AI / Machine learning
We have leading expertise in developing and validating QSAR models.
We apply our machine learning expertise to the development of models in SaferSkin™ applications including customized versions for customers.
Our AI team has competency in extracting targeted knowledge from information collections (documents, abstracts etc.) and organizing it as structured information e.g., available to applications from a customized EdelweissData™ instance.
Data Management
Our EdelweissData™ approach to data management organizes all data within our system using best data practices and standards.
All metadata and data is harmonized and aims for completeness and integrity.
Data processing workflows are organized as reproducible in silico protocols with instances stored within the system for future reference.
All in silico and in vitro protocols are fully described and stored in the system.
Secure customer data management solutions can be provided through premium customer accounts on a secure cloud.
Partner contribution
Together with our partners we serve you with up-to-date in vitro assays, computational toxicology and PBPK methods, consumer testing, and regulatory toxicology assessments. Our mission and expertise is to ensure consumer safety by identifying potential dangers in your chemical formulations and evaluating the safety of your finished products.
We will provide step-by step assistance for your projects in cosmetic and pharmaceutical development – while maintaining full compliance with all regulatory requirements. At your disposal is a diverse portfolio of assays and methods, ranging from the well-established to the most cutting-edge.
All techniques meet the stringent criteria of modern science and are performed to the highest professional standard. We are devoted to providing an excellent, transparent and personalized service.
The partner contribution includes:
PKDERM
PKDERM if a French company which was founded in 2018. It offers a wide range of newest in vitro assays and models for skin safety assessment. Their focus lies on products applied in the fields of cosmetics, dermatology, pharmacy, chemicals, agro/petrochemicals and medical devices.
> Read more
SenzaGen
SenzaGen is a Swedish biotech company that provides state-of-the-art non-animal tests for assessing a substance’s allergenicity. The GARD® test method combines genomic data from human cells with machine learning for a unique capability to identify and analyze whether a chemical could cause allergic reactions on the skin or in the respiratory tract.
> Read more
Curio Biotech
Founded in 2017, Curio Biotech Ltd is a GLP ready Swiss Contract Research Organization (CRO) located at BioArk (biotech park), Visp, Switzerland. It provides in vitro testing services to cosmetic, pharma (small and large molecules) and diagnostic industries using proprietary culture media and primary human cell based 3D co-culture models. In vitro services focus on basic research, drug discovery, toxicity, pharmacology in the areas of dermatology, immuno-oncology and regenerative medicine. Furthermore, Curio Biotech offers a variety of batch/lot release tests like potency tests for bio-pharma industry, biocompatibility tests and other regulatory OECD tests.
Biopredic
Biopredic International is a French privately-owned biotechnology company founded in 1993. Biopredic’s expertise was initially based on primary hepatocyte cryo-preservation and quickly grew to encompass the isolation, production, and distribution of fresh and frozen human and animal biological products, including tissues, primary cells, cell lines, and reagents.
> Read more
Eurosafe
Eurosafe is a GLP-certified (A level) Contract Research Organisation (CRO), specialised in the testing of human and animal pharmaceutical products, cosmetics (ingredients and finished goods), food supplements, medical devices and biocides. Eurosafe provides state-of-the-art in-vitro ADME tests for chemical compounds, safety tests, in-vivo efficacy and tolerance studies, toxicological expertise as well as consulting services on the European regulations. Eurosafe is one unique CRO that covers several services organized around 3 specialties: 1. In vitro assays. 2. Tests on volunteers. 3. Toxicological expertise and regulations.
> Read more
React4Life
React4life is an Italian biotech company founded in 2016 after more than 15 years of R&D by Silvia Scaglione and Maurizio Aiello, together with a multidisciplinary team of experts, with the ambition to turn the promise of biomedical research into concrete benefits for society. Scientists can finally rely on a new enabling technology to recapitulate human biology in the lab: MIVO® (Multi In Vitro Organ), the next generation of organ-on-a-chip. This cutting-edge microphysiological system enables researchers to overcome the limits of current in vitro assays and animal models. It improves the reliability and predictivity of several applications: pharmaceutical drug-testing, personalized medicine, novel immuno-oncology therapies, cosmetics, dermatology, and nutraceuticals.
> Read more
EdelweissConnect
Edelweiss Connect (EwC) - as the provider of this SaferWorldbyDesign platform - offers the expertise and experience to initiate, coordinate and manage large collaborative research projects, with partners from industry, government, and academia. Our goal is to incubate high-impact products, services, and solutions at the forefront of innovation, with sustainability and responsibility.
> Read more
In vitro testing
In vitro sensitization assay testing according to OECD guidelines and GLP standards
Our laboratories historically specialize in in vitro studies. We offer a portfolio of assays ranging from the well-established to the most cutting-edge, guided by in silico modelling.
Some assays are regulatory compliant to ensure safety and tolerance of finished cosmetic products. We can evaluate dermal absorption and delivery of a test substance using excised skin.
In order to determine the possibility of peripheral effects during dermal administration of test compounds, it is essential to evaluate the potential of transcutaneous passage of compounds. Our in vitro methods can measure the diffusion of chemicals across different skin models. Transcriptomics experiments can reduce the uncertainty in assay results using mechanistic gene signature analysis.
Available assays are
SaferSkin Formulation testing
With the SaferSkin Formulation testing solution we provide:
- Strong business cases supporting value gains for data-driven decisions supported by weight of evidence.
- Evaluation of uncertainty in current assessment based on Bayesian Network and AI methods.
- Business Case workflow for use of New Approach Methods (NAMs), Integrated Approaches to Testing and Assessment (IATAs) and Read Across to reduce uncertainties in recommended safe level of use of ingredients in formulation.
- Integrated Testing of key ingredients and formulations available in workflows orchestrating remote testing and return of data, models and reports to system.
SaferSkin Case Studies
SaferSkin successfully provides skin sensitization assessment using advanced Defined Approaches (DA) such as Bayesian Networks, regression, and 2 out of 3 Weight of Evidence (WoE). We also work with partners in a process to include advanced assays such as SENS-IS and GARD assays. Using SaferSkin characterization of chemicals can be achieved using different approaches and comparative information is provided for better decision making and risk assessment. Here are several case studies where we compared the results of different approaches including DAs from OECD TG 497.
> Click here for SaferSkin Case Studies
Prediskin
Protocols
Prediskin is able to assess the potential cutaneous irritation of a cosmetic product related to its toxic effect on skin cells and its condition of exposure, using fresh human skin discs.
> Learn more.
Data Analysis
A notebook which demonstrates how Prediskin assay data published in the EdelweissData can be accessed and analysed.
> Learn more.
Prediskin Assay
To ensure transparency and reproducibility of data management in a Prediskin assay we employed our EdelweissData solution.
> Learn more.
Prediskin Shop
Order Skin disks, in vitro or in vivo assays, Regulatory toxicology services.
> Learn more.
The SaferSkin Application
The SaferSkin application is a web application, which is used for predicting ingredient’s potential to cause skin sensitisation, without animal testing. It applies integrated strategies of safety assessment to skin sensitisation and combines the top three approaches for accurate predictions of skin sensitisation.The application applies state-of-the-art science to workflows over the entire discovery and development funnel, to regulatory acceptance and registration. For more information see the «SaferSkin application» section on this page.
The SaferSkin Application
- The SaferSkin application is a web application, which is used for predicting ingredient’s potential to cause skin sensitisation, without animal testing.
- The application applies integrated strategies of safety assessment to skin sensitisation.
- It combines the top three approaches for accurate predictions of skin sensitisation.
- The application applies state-of-the-art science to workflows over the entire discovery and development funnel, to regulatory acceptance and registration.
Check the science behind the app > here.
The app can be used for:
- Hazard identification, classification, and labeling under the Globally Harmonised System of Classification and Labelling of Chemicals (GHS) scheme (UN 2013).
- Quantitative risk assessment (QRA) especially when combined with in vivo evidence on analogs.
- Development of an efficient testing strategy, as a decision strategy tool.
Main features
- Comparison of 3 approaches: Bayesian Network, ‘2 out of 3 weight of evidence’, multiple regression
- Category prediction with confidence levels
- Simple to use interface
- Single compound input submission - entered as SMILES or drawn
- Physical properties calculated
- LLNA pEC3 & EC3 values from 50-90th percentile confidence
The application
> Read more
> Read more
Regulatory framework
To ensure scientific integrity we apply our services under the newest authority regulations. With this we operate under the highest ethical standards to guarantee results with highest value.
Skin Sensitisation
- OECD GuidelineNo. 497 on Defined Approaches for Skin Sensitisation
- OECD Test Guideline No. 442C In Chemico Skin Sensitisation
- OECD Test Guideline No. 442D In Vitro Skin Sensitisation
- OECD Test Guideline No. 442E In Vitro Skin Sensitisation
Skin Irritation
- OECD TG 439
Skin Permeation
- OECD TG 428
Phototoxicity
- OECD TG 432
- OECD TG 498
Reference:EwC-SS-1-PP
Vendor:Edelweiss Connect, Switzerland
Product: SaferSkin Service Premium Package
Reference:EwC-SS-3-SP
Vendor:Edelweiss Connect, Switzerland
Product: SaferSkin Service Silver Package
Reference:EwC-SS-4-BP
Vendor:Edelweiss Connect, Switzerland
Product: SaferSkin Service Bronze Package
Reference:EwC-SSR-1
Vendor:Edelweiss Connect, Switzerland
Product: SaferSkin Sensitisation Report (1)
Reference:EwC-SSR-2
Vendor:Edelweiss Connect, Switzerland
Product: SaferSkin Sensitisation Report (2)
Reference:EwC-SSR-3
Vendor:Edelweiss Connect, Switzerland
Product: SaferSkin Sensitisation Report (3)
Reference:TRA2T2416
Vendor:Biopredic International
Product: Frozen Human dermatomed Skin OECD guideline compliant
Reference:TRA2T2418
Vendor:Biopredic International
Product: Frozen Human dermatomed Skin OECD guideline compliant
Reference:TRA2T2420
Vendor:Biopredic International
Product: Frozen Human dermatomed Skin OECD guideline compliant
Reference:TRA2T24R1
Vendor:Biopredic International
Product: Frozen Human dermatomed Skin OECD guideline compliant
Reference:TRA2T24R2
Vendor:Biopredic International
Product: Frozen Human dermatomed Skin OECD guideline compliant
Reference:TRA2T24R3
Vendor:Biopredic International
Product: Frozen Human dermatomed Skin OECD guideline compliant
Reference:TRA2T24R4
Vendor:Biopredic International
Product: Frozen Human dermatomed Skin OECD guideline compliant
Reference:TRA2T4716
Vendor:Biopredic International
Reference:TRA2T4718
Vendor:Biopredic International
Reference:TRA2T4720
Vendor:Biopredic International
Reference:TRA2T47R1
Vendor:Biopredic International
Reference:TRA2T47R2
Vendor:Biopredic International
Reference:TRA2T47R3
Vendor:Biopredic International