The SaferWorldbyDesign Blog

SaferWorldbyDesign's expertise in data science combines technical expertise with strategic thinking, identifying key data sources to analyze and interpret the data and developing predictive models to mitigate potential chemical risks.

SaferWorldbyDesign - We will be participating in the Society of Toxicology (SOT) Annual Meeting and ToxExpo in Nashville!

Recently, the FDA Modernization Act 2.0 became law in the US, and crucially included a provision that ends the requirement of animal testing for new drugs.

ToxCalc! is a website purpose-built for toxicologists and other scientific professionals to help us do routine calculations.

Born in 2008 as a dedicated REACH consulting company, TEAM mastery now provides a range of services in Chemical Regulatory Affairs.
The term Knowledge Graph describes a new type of database technology that is applied to store data about a specific domain and to query the database with questions in natural language.

RISK-HUNT3R, short for “RISK assessment of chemicals integrating HUman centric Next generation Testing strategies promoting the 3Rs"

SenzaGen today announced that the Company has obtained approval from the OECD for GARD®skin as a test guideline for non-animal skin sensitization.

We are proud to announce that the catalog of SaferWorldbyDesign products and services is now available through both the Science Exchange and Scientist.com networks.

This internship program is organised by Edelweiss Connect, GmbH, and SaferWorldbyDesign partners. Internships will run for six months with an emphasis on virtual work. Opportunities to visit and be hosted by SaferWorldbyDesign partners will also be considered.
The need to reduce, refine and replace animal experimentation has led to a boom in the establishment of new approach methodologies (NAMs). This promising trend brings the hope that the replacement of animals by using NAMs will become increasingly accepted by regulators, included in legislation, and consequently more often implemented by industry.

Reliable analysis and consumption of data should be based on a foundation of sound data preparation, well-prepared metadata, data processing protocols, and reliable exposure of data through interfaces to end-user applications for data analysis, risk, and safety assessment.
Skin sensitization assessments for chemicals, cosmetics, and many pharmaceuticals are required by regulatory bodies for hazard assessment and approval. In June 2021, the Organization for Economic Co-operation and Development (OECD) published the new guideline (TG 497) for skin sensitization.

An important question that was addressed within the EU-ToxRisk project relates to the extrapolation from acute to chronic effects of toxicants.
Nonalcoholic fatty liver disease (NAFLD), the most common cause of chronic liver disease worldwide, is rapidly becoming the predominant cause of end-stage liver disease and a leading indication for liver transplantation.

This blog post presents a collection of perspectives that elaborate our vision of reliable and reproducible science supporting product and risk assessment as presented by us this year at the Precision Health conference co-organized by MCBIOS and MAQC.

The SaferSkin™ Sensitization application implements three well-adopted defined approaches to perform an integrated analysis of the OECD-approved in vitro tests (DPRA, KeratinoSens™, h-CLAT) for assessment of skin sensitisation potential.

The recent OECD acceptance of TG 497 for defined approaches for skin sensitization marks an important milestone.
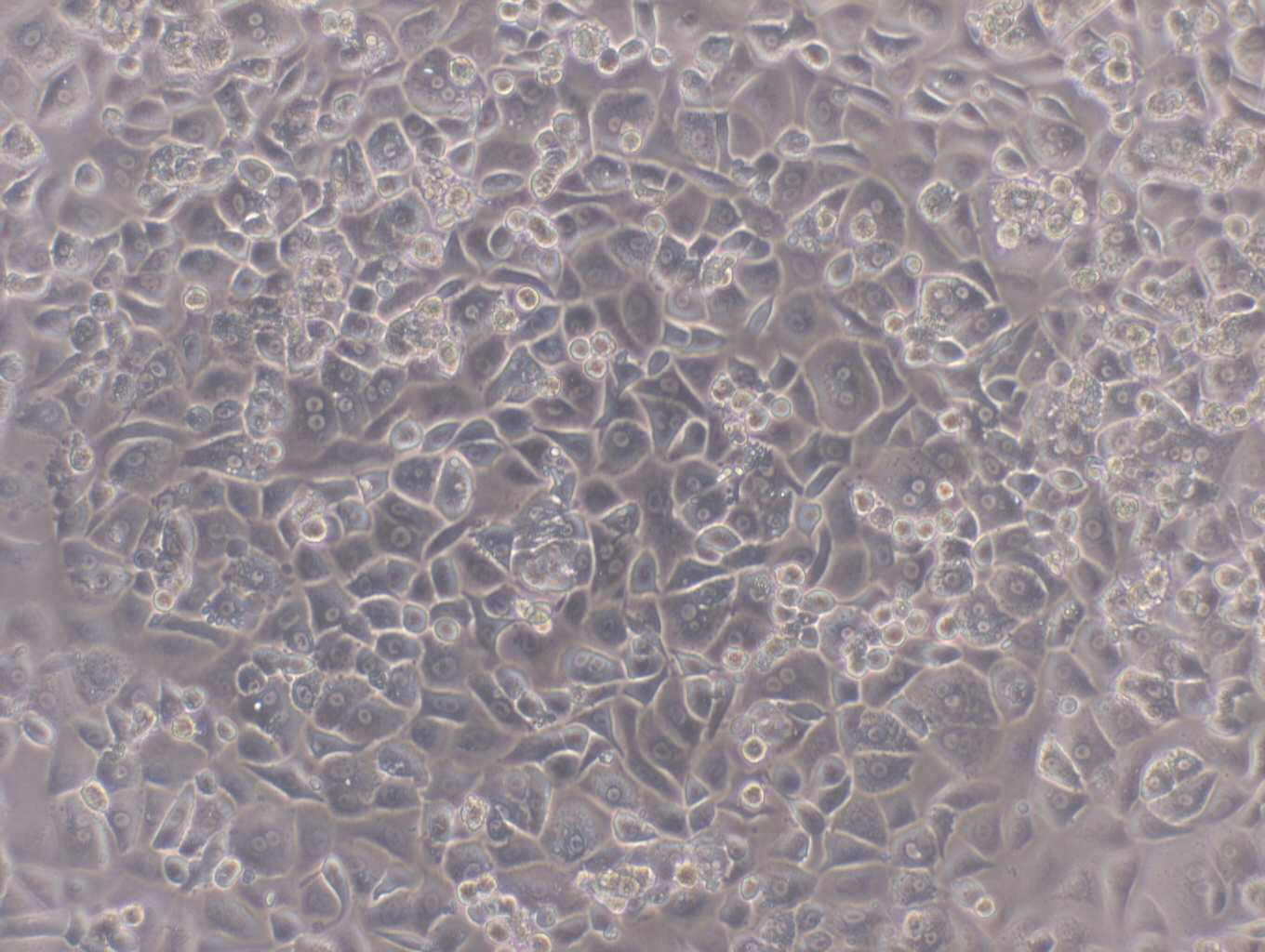
HepaRG™ has been manufactured and distributed for many years by Biopredic International, now part of Wepredic. We realized in recent years the increasing need for the development of HepaRG™ infrastructure and services to keep up with the increasing requirements of the HepaRG™ research community and requests from our customers.

A better molecular characterization of a cell line is a prerequisite for well-planned, educated, and targeted experimentation.

An important commitment we are making is to ensure data integrity across the journey from research to practice, from case study work to service execution, from study planning through data generation to downstream consumption.

This innovative method (Su et al. 2016) was awarded with the Lush Science Price 2016 and combines cell-based assays with bioinformatics. The method predicts specifically toxicity to renal proximal tubular cells, which are one of the main targets for chemical-induced toxicity in the kidney.

Drug-induced liver injury (DILI) accounts for more than 50% of acute liver failure and constitutes therefore a major concern for the pharmaceutical industry in drug pre-and post-marketing scenarios.
For many years HepaRG™ has provided a valuable liver cell line for research applications.

Data is the gold of scientific prospecting, whether supporting research publications in academia or product development in industry.

The EU-ToxRisk Commercialisation Platform was launched at the EU-ToxRisk Stakeholder meeting in Egmond, the Netherlands on 12 February.

Skin safety is one of the most important goals of the consumer products industry.

Taxila™ is a platform that aggregates the tons of data produced every day in natural text and converts it to actionable insights.

Dr. Christophe Chesné, the founder and CEO of Biopredic International and Eurosafe, explains why his two companies are founding partners of SaferWorldbyDesign.

Co-founder and CEO Tom Bell explains why Vertex Laboratories is a founding member of SaferWorldbyDesign.